DAT General Chemistry Review
This online course video tutorial review focuses on the general chemistry section of the DAT Exam – the Dental Admission Test. It provides plenty of notes along with the fundamental concepts of topics such as matter, stoichiometry, chemical reactions, and gas law chemistry. It contains a list of equations, formula sheets, along with explanations on how to use the formulas the right way. This study guide contains plenty of examples and practice questions and hard problems for you to do well on the DAT exam.
Full DAT Course Review:
https://vimeo.com/ondemand/datreviewc...
Here is a list of topics:
1. Atoms, Molecules and Ionic vs Molecular Compounds
2. Pure Substances vs Heterogeneous and Homogeneous Mixtures
3. Ionic vs Covalent Bonding plus Coordinate Covalent Bonds
4. Isotopes, Allotropes, Alloys, Intensive and Extensive Properties
5. Characteristics of Metals, Metalloids, and Nonmetals
6. Periodic Table – Alkali Metals, Alkaline Earth, Chalcogens, Halogens, and Noble gases
7. Density Practice Problems, Unit Conversion, Dimensional Analysis and the Metric System
8. Temperature Conversions, Celsius, Kelvin and Fahrenheit
9. Grams, Moles, Atoms, Particles, Ions, Molecules, and Formula Unit Conversions
10. Mole to Mole and Gram to Gram Stoichiometry
11. Molar Mass, Atomic Mass, Molecular Weight and Formula Weight Calculations
12. Average Atomic Mass and Relative Percent Abundance of Isotopes
13. Phases of Matter – Solids, Liquids, Gases, and Plasma
14. Actual Yield, Theoretical Yield, and Percent Yield Calculations
15. Mass %, Percent Composition, and Empirical Formula Determination – Combustion Analysis
16. Limiting and Excess Reactant Stoichiometry Problems
17. Synthesis / Combination and Decomposition Reactions
18. Oxidation Numbers and States for Elements, Compounds, and Polyatomic Ions
19. Nomenclature of Ionic Compounds, Molecular Compounds, and Acids
20. Polar and Nonpolar Covalent Bonds
21. Redox Reactions – Oxidation vs Reduction – How to Identify The Oxidizing and Reducing Agent
22. Single Replacement and Double Displacement Reactions Including Combustion Reactions
23. Precipitation, Acid-Base Neutralization, and gas evolution reactions
24. Accuracy vs Precision & the activity series of metals
25. Molarity Calculations, Acid Base Titrations, and Dilution Problems
26. Solution Stoichiometry with Limiting and Excess Reactant
27. Net Ionic Equations and Spectator Ions
28. Strong, Weak, and Nonelectrolytes
29. Solubility Rules – Soluble vs Insoluble Compounds
30. How to Write the Formula for Ionic and Covalent Compounds Including Acids
31. Strong Acids, Weak Acids, Strong Bases, and Weak Bases
32. Acidic, Basic, and Neutral Salts
33. Arrhenius, Bronsted Lowry and Lewis Acids and Bases
34. Gas Laws – Ideal Gas Equation and the Combined Gas law formula
35. Boyle’s, Charles, Gay Lussac, and Avogradro’s Law Equations
36. Mole Fraction, Partial and Total Pressure
37. Dalton’s Law of Partial Pressures and Graham’s Law of Effusion
38. Gas Density at STP and Molar Mass Problems
39. How To Identify The Unknown Gas
40. Gas Stoichiometry Problems with Liter to Liter Conversions
41. Average Kinetic Energy of a Gas and Temperature
42. Root Mean Square Velocity of a Gas
43. Kinetic Molecular Theory of Ideal Gas
44. Temperature and Pressure Conditions For Ideal Gases
-
 20:21
20:21
Math Easy Solutions
25 days ago $0.18 earnedImportant Chemistry Terms
1241 -
 2:19:07
2:19:07
TheOrganicChemistryTutor
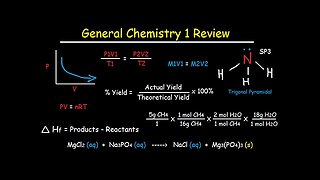
6 months agoGeneral Chemistry 1 Review Study Guide - IB, AP, & College Chem Final Exam
390 -
 19:31
19:31
Math Easy Solutions
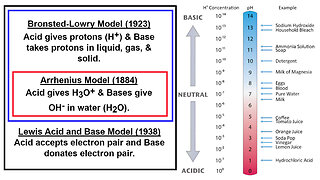
29 days ago $0.07 earnedAcids and Bases: Brønsted–Lowry, Arrhenius, and Lewis Models
1621 -
 55:13
55:13
Human Consciousness Support (Official)
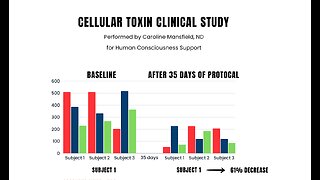
4 months agoDr. Robert Young & Caroline Mansfield Discuss a Protocol Removing Forever Chemicals Including Graphene Oxide.
4.1K1 -
 16:08
16:08
FanaticVoyage
2 months agoExperiments with the Bubble Model of Metal Structure 1952 - Sir Lawrence Bragg, W.M Lomer, J.F. Nye
59 -
 5:34
5:34
robdefarias
7 months agoExercício 16.37 de "General Chemistry", 11ª ed., Ebbing-Gammon
1 -
 3:04
3:04
robdefarias
7 months agoExercício 17.31 de "General Chemistry", 11ª ed., Ebbing-Gammon
3 -
 1:45
1:45
mycustomessays
7 months agoChemistry homework help
6 -
 2:26
2:26
FanaticVoyage
2 months agoEquilibrium NaCI Crystal Formation By RH Rate Changes - Short Educational Clip
101 -
 2:14
2:14
scamornoreviews
1 month agoInstant Chemistry Formula Review | Does Instant Chemistry Formula Really Work?
91